Detection and quantitation of Hepatitis B virus (HBV) in less than one hour
VIDEO
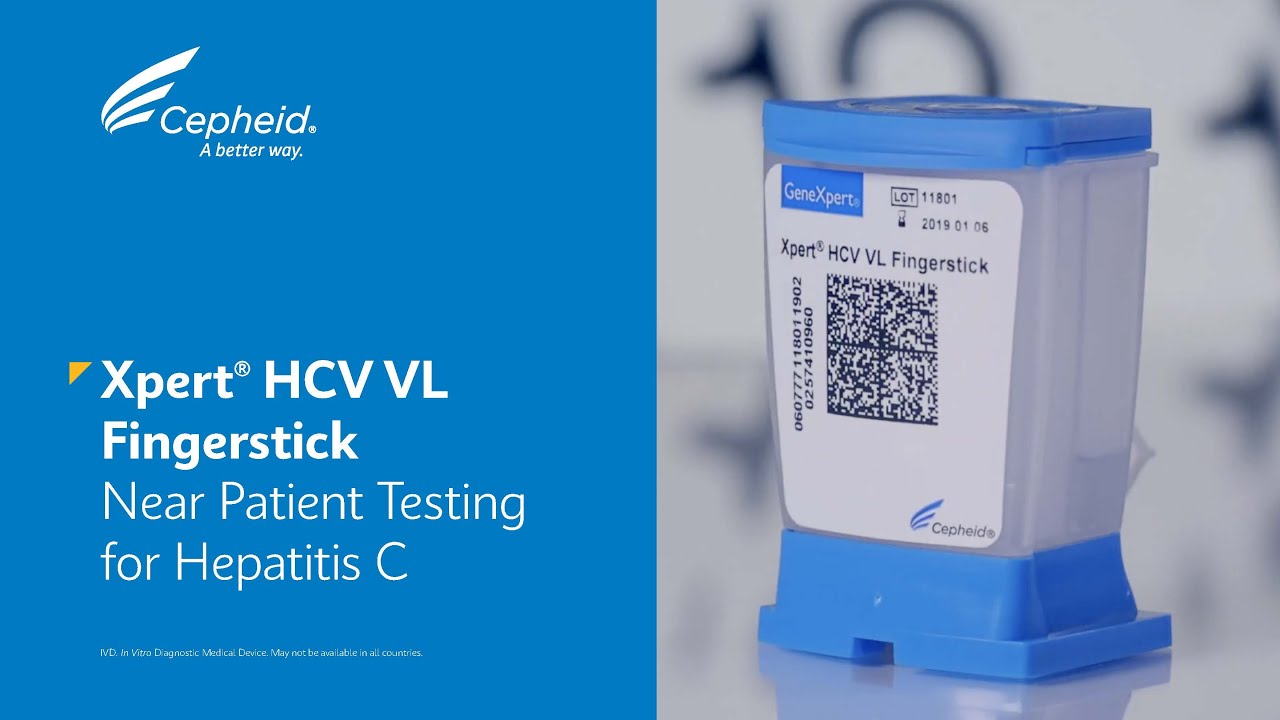
London Pharmacy Video - Xpert HCV VL Fingerstick - Near Patient Testing for Hepatitis C
The WHO has set a goal to eliminate Hepatitis C by 2030. England will strive for 2025.